news
-
ISO 8 cleanroom
An ISO 8 cleanroom is a controlled environment designed to maintain a specific level of air cleanliness and is widely used in industries like pharmaceuticals, biotechnology, and electronics. With a maximum of 3,520,000 particles per cubic meter, ISO 8 cleanrooms are classified under the ISO 14644...Read more -
What is a Cleanroom Panel? Comprehensive Guide
Cleanroom panels are an essential component of controlled environments, such as cleanrooms, where contamination control is critical. These panels are typically made of prefabricated materials, such as galvanized steel or aluminum, and are designed to create a seamless, airtight barrier that preve...Read more -

What is cleanroom
A cleanroom is a controlled environment designed to maintain extremely low levels of particulate matter such as dust, airborne microorganisms, aerosol particles and chemical vapors. These controlled environments are critical to industries such as pharmaceuticals, biotechnology, electronics, and ...Read more -
Everything You Need to Know About Cleanroom Panels
Cleanroom panels are an essential component of controlled environments, such as cleanrooms, where contamination control is critical. These panels are typically made of prefabricated materials, such as galvanized steel or aluminum, and are designed to create a seamless, airtight barrier that preve...Read more -

How to disinfect the workshop at different levels of cleanrooms
The disinfectant combination scheme used in the grade A area is the strategy of using sterile and non-residual disinfectants, and alcohols are generally selected. Such as 75% alcohol, IPA or complex alcohol. It is mainly used for disinfection o...Read more -

Welcome to visit us in CPHI PMEC Shanghai 2024!
CPHI & PMEC China is Asia’s leading pharmaceutical show for trade, knowledge sharing, and networking. It covers all industry sectors along the pharmaceutical supply chain, providing a one-stop platform to grow your business in the world’s second-largest pharma market. The growing internationa...Read more -

temperature and humidity control of laboratory cleanroom
Laboratory temperature and humidity monitoring is very important because the temperature and humidity in the laboratory may affect the results of experiments and the use of instruments. Generally speaking, temperature and humidity monitoring in the laboratory mainly incl...Read more -

Application of FFU
FFU (Fan Filter Unit) is a device used to provide a highly clean environment, often used in semiconductor manufacturing, biopharmaceuticals, hospitals and food processing where a strictly clean environment is required. The use of FFU FFU is widely used in a variety of environments requiring high...Read more -
The weight of the color steel plate and the weight per square meter
Load-bearing and self-weight parameters of clean panel: Clean panel per square meter bearing: 1. Single-sided glass magnesium manual plate (0.476mm)— -150kg 2. Double-sided glass magnesium manual plate (0.476mm)— -150kg 3. Double-sided glass magnesium machine-made board (0.476mm)̵...Read more -
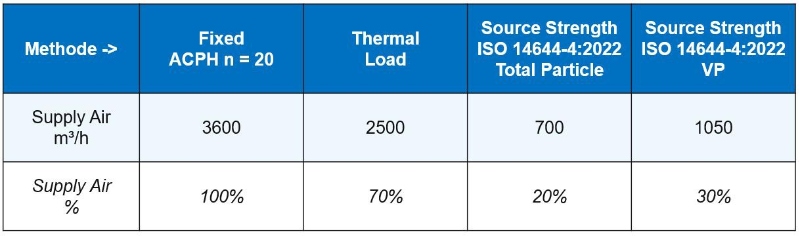
Clean room wind speed requirements and air changes
Sufficient ventilation volume is to dilute and eliminate indoor polluted air, according to different cleanliness requirements, when the clean room net height is higher, the appropriate increase in the number of air changes. Among them, the ventilation volume of 1 million...Read more -
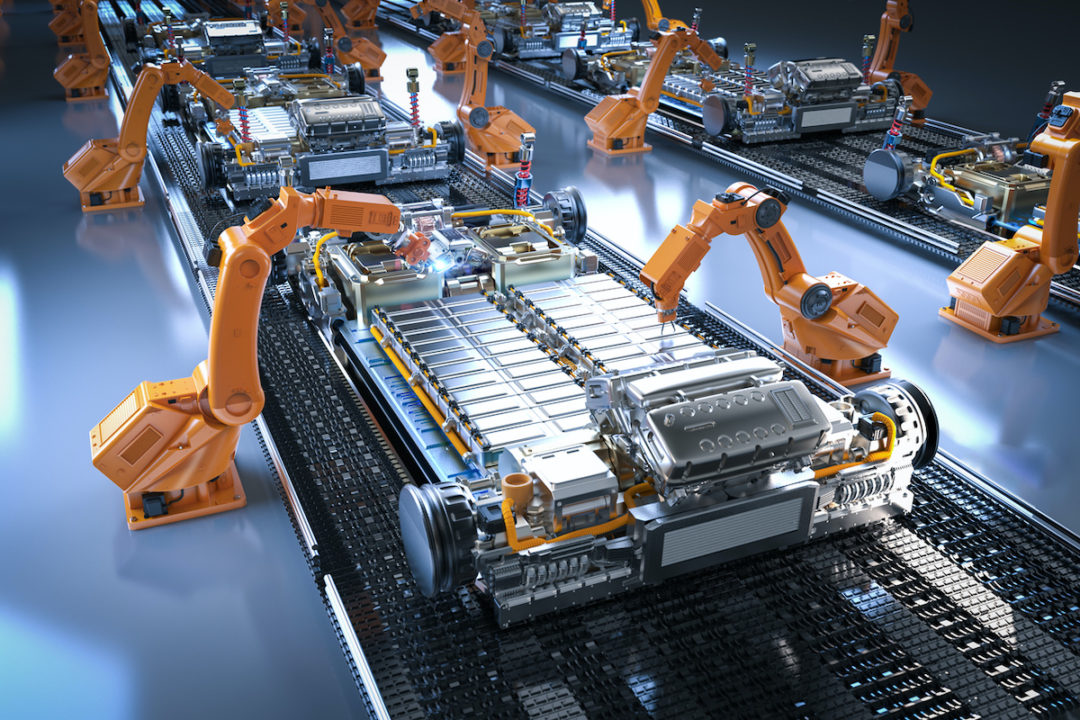
Production of new energy car in clean room
It is understood that a complete car has about 10,000 parts, of which about 70% are carried out in the clean room (dust-free workshop). In the car manufacturer’s more spacious car assembly environment, the oil mist and metal particles emitted from the robot and other assembly equipment will...Read more -

Requirements of medical clean room
The first point of clean room design is to control the environment. This means ensuring that the air, temperature, humidity, pressure and lighting in the room are properly controlled. The control of these parameters needs to meet the following requirements: Air: Air is one of the most important f...Read more





 Home
Home Products
Products Contact Us
Contact Us News
News